Understanding VAERS: Post-Vaccine Deaths and Reporting Issues
Written on
Chapter 1: The Complexity of Vaccine Safety
Vaccine safety is a contentious subject, often polarized into two camps: those who advocate for their safety and those who warn against them. However, the reality tends to be more nuanced. Vaccines, like many pharmaceuticals such as antibiotics or antidepressants, come with potential risks but also aim to provide significant benefits.
This article seeks to evaluate the claims surrounding the safety of Covid-19 vaccines as reflected in the Vaccine Adverse Event Reporting System (VAERS), a passive monitoring system designed to assess vaccine safety beyond the confines of clinical trials.
The Narrative of Unsafe Covid-19 Vaccines
In a widely discussed episode of the DarkHorse podcast, a trio comprised of Bret Weinstein, Ph.D., Robert Malone, MD, MS, a pioneer in mRNA technology, and Steve Kirsch, MS, raised concerns regarding how the pandemic has been managed, particularly focusing on vaccine safety data from VAERS.
They presented a graph that illustrated a notable increase in post-vaccine deaths in 2021 compared to prior years in the U.S., suggesting that the rise could be linked to Covid-19 vaccinations. Notably, in May 2021, Tucker Carlson echoed similar claims during his show, citing nearly 4,000 deaths following vaccination, based solely on VAERS data.
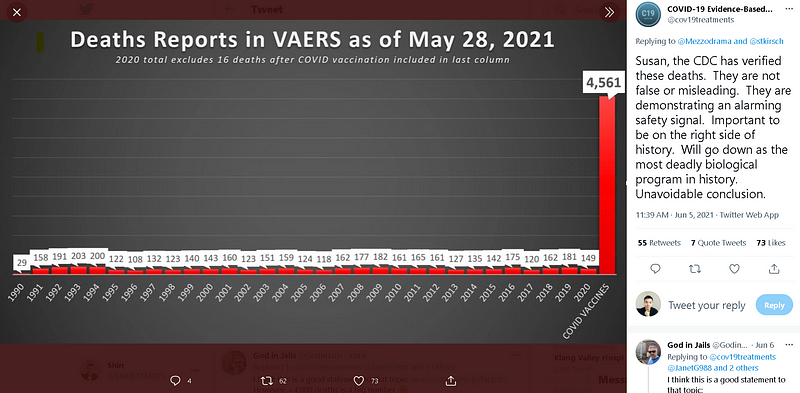
The graph referenced by Mr. Kirsch indicated that the reported figures might be significantly understated. He posited that the actual number of vaccine-related deaths could be much higher, suggesting that only a small fraction of adverse events are reported. For example, he speculated that if 5,000 reports exist, the true number could be closer to 500,000, though he deemed that estimate excessive. He argued that even 5,000 deaths would surpass all deaths reported for 70 other vaccines over the past three decades.
Mr. Kirsch further claimed that the VAERS system is not widely understood, leading to underreporting, even suggesting that healthcare professionals are discouraged from reporting adverse events.
The Role of VAERS in Vaccine Monitoring
To grasp the issue of underreporting and post-vaccine fatalities, it’s essential to understand the intended function of VAERS. Established in 1990, VAERS was designed for the CDC and FDA to monitor vaccine safety in the U.S. population beyond clinical trials, given that trial participants do not fully represent the general populace.
For instance, the Pfizer mRNA vaccine trials had a median participant age of 52, with only a small percentage of older individuals and those with various underlying health conditions. This exclusion can limit the generalizability of trial results, necessitating real-world data collection through systems like VAERS.
It’s important to note that without a comparative control group, VAERS cannot conclusively determine whether a vaccine caused any adverse event. As outlined by the CDC, reports to VAERS may be incomplete or inaccurate and cannot alone establish causation.
The primary purpose of VAERS is to identify potential signals of adverse events that warrant further investigation.
VAERS Underreporting: The Facts
VAERS allows anyone, including patients and healthcare providers, to submit reports. However, underreporting remains a recognized limitation. Critics, including Mr. Kirsch, often assert that only 1% of vaccine-related adverse events are reported, a claim stemming from a 2011 Harvard Pilgrim Health Care study.
While this figure is frequently quoted, it can be misleading. The Harvard study encompassed all reactions, including mild side effects that many consider trivial. More severe adverse events, however, tend to be reported at higher rates.
For example, a 1995 study revealed that 68% of severe cases of poliomyelitis associated with the oral poliovirus vaccine were reported to VAERS. In contrast, less severe reactions, such as rashes from the MMR vaccine, fell below 1%.
More recent studies have shown variable reporting sensitivities for various severe reactions, such as anaphylaxis and Guillain–Barré syndrome. Generally, less severe reactions are often underreported, while serious events are more likely to be captured.
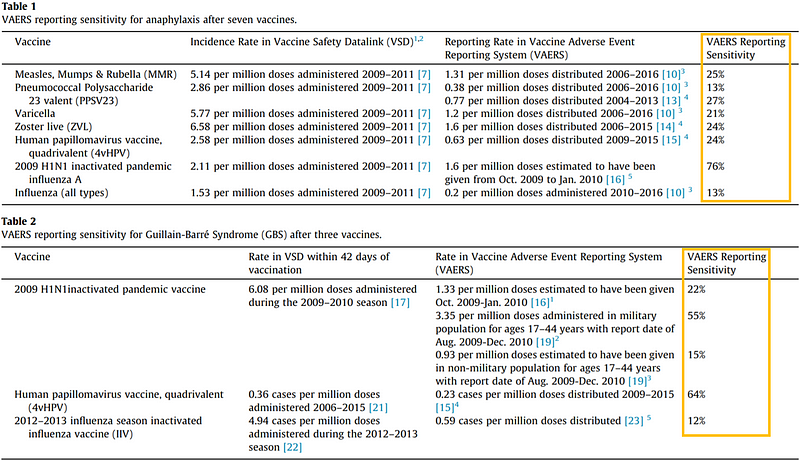
Despite the inevitability of underreporting within VAERS, this does not inherently render vaccines unsafe. The context of Covid-19 vaccination reveals that while certain adverse events may be underreported, serious reactions remain relatively rare.
Post-Vaccine Deaths: Contextualizing the Data
The aforementioned podcasts and reports highlight alarming figures, such as 4,000 to 5,000 reported deaths following Covid-19 vaccinations as of May 2021. However, these numbers can be misleading without context. During this timeframe, approximately 250 million Covid-19 vaccine doses were administered, yielding a post-vaccine death rate of around 0.002%.
In fact, the CDC anticipated 11,440 deaths among the 1.3 million doses administered in long-term care facilities, yet only 129 deaths were reported to VAERS, a figure significantly lower than expected.
As of July 13, 2021, the CDC indicated approximately 6,000 post-vaccine deaths out of 334 million doses administered, resulting in a rate of 0.0018%.

Given the massive scale of vaccination efforts, a rise in reported adverse events is not surprising. Moreover, VAERS is not intended to establish causation; it simply collects data, which can lead to misconceptions about vaccine safety.
Evaluating the Utility of VAERS
Despite its limitations, VAERS has proven valuable in identifying potential safety signals. For instance, a 2001 study prompted actions to withdraw the rotavirus vaccine after detecting adverse events. Similarly, VAERS played a role in identifying risks associated with the AstraZeneca and Johnson & Johnson vaccines, leading to temporary suspensions.
Furthermore, reports of myocarditis linked to mRNA vaccines in young men and the association between the Johnson & Johnson vaccine and Guillain–Barré syndrome were initially flagged by VAERS, leading to recognized risks.
In conclusion, while VAERS may not capture all adverse events, it remains a crucial system for monitoring vaccine safety. Critics may argue that it underreports certain events, but the detected adverse events are still significant, as even rare occurrences can prompt vital public health responses.
Key Points
- While underreporting is an issue in VAERS, the extent varies by the severity of adverse reactions.
- Serious reactions are more likely to be reported than minor ones.
- The rate of post-vaccine deaths must be contextualized against the number of vaccine doses administered.
- VAERS serves an essential role in monitoring vaccine safety and has led to important investigations and responses.
A clear explanation of the VAERS mRNA death curve and its implications.
An overview of the Vaccine Adverse Event Reporting System (VAERS) and guidance on reporting.