# A Programmable Epigenetic Editor: Turning Genes On and Off
Written on
Chapter 1: Understanding Gene Expression
Within our cells lies the complete genetic blueprint necessary to create a human being. Most of this genetic material resides in the nucleus of the cell, with some fragments located in the mitochondria. Among this genetic material are genes, which are sequences that code for proteins. These sequences undergo transcription to form messenger RNA, which is subsequently translated into amino acids. When linked together, these amino acids create proteins. Additionally, certain genes known as transcription factors can influence the activity of other genes.
This modulation of gene activity is a significant factor in why different cell types—despite sharing the same genome—exhibit distinct appearances and behaviors.
To complicate matters further, we must consider the field of epigenetics.
Section 1.1: The Role of Epigenetics
Epigenetics is the study of modifications that can be added to or removed from DNA. These epigenetic changes can interact with transcription factors, adding another layer of complexity to gene regulation. As we delve deeper into genetics, it's becoming increasingly clear that not only do genes matter, but so does how they are expressed. For example, in aging brains, alterations in gene activity lead to various signs of aging.

Section 1.2: The Discovery of CRISPR
CRISPR, short for clustered regularly interspaced short palindromic repeats, refers to specific DNA sequences first discovered in bacteria in 1987. These sequences are characterized by their short, repeated nature and their palindromic structure. Initially thought to be a curiosity of the genome, their true significance became apparent in 2007 when scientists realized that these sequences help bacteria fend off viral infections. By incorporating segments of viral DNA into their own genome, bacteria can recognize and combat subsequent infections from the same virus.
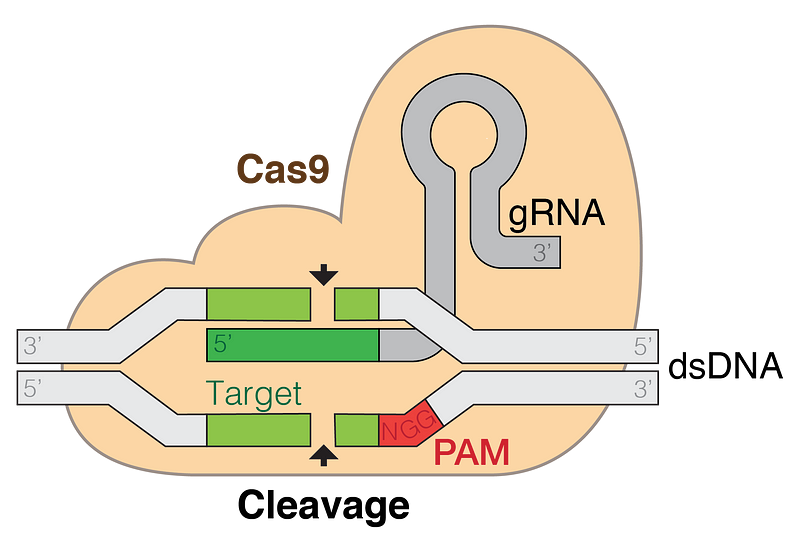
In 2012, a major breakthrough occurred when researchers recognized the potential of these sequences for gene editing. Scientists such as Jennifer Doudna and Emmanuelle Charpentier, along with Feng Zhang's team, developed CRISPR-based tools that utilize the DNA-cutting protein Cas9. This innovation has made gene editing faster, more cost-effective, and more precise than ever before.
Chapter 2: Introducing Epi(c) CRISPR
What if we could apply CRISPR technology not to alter DNA but to modify the epigenome? Instead of cutting DNA, we could simply tag it. Often, the goal is to turn genes on or off, ideally in a reversible manner—a challenge with traditional CRISPR methods.
Enter CRISPRoff, a groundbreaking innovation that functions as a programmable epigenetic memory writer. This system employs a modified form of the Cas9 protein, rendered "dead" to prevent DNA cutting. Paired with single guide RNAs that target specific genes, the CRISPRoff system can attach epigenetic methyl groups to genes, influencing their activity without changing the underlying DNA sequence.
To validate their system, researchers tested it on pluripotent stem cells, successfully silencing a gene and observing this change persist through differentiation into neurons. In another experiment, they reduced the expression of the Tau protein, which is associated with Alzheimer's disease, demonstrating a significant decrease in expression without completely silencing the gene.
The authors of the study highlight the extensive potential of CRISPRoff, suggesting its capability for diverse applications, including genome-wide screenings, enhanced cell engineering, and exploring the mechanisms of epigenetic inheritance.
While this initial study serves as proof of concept, it presents exciting possibilities in the field of epigenetics.