# The Race for Coronavirus Vaccines: 7 Leading Candidates Revealed
Written on
The Current Landscape of COVID-19 Vaccines
The development of vaccines is progressing rapidly, with over 200 candidates in various stages and seven currently in Phase 3 trials. This gives us hope in our battle against COVID-19.
But when can we expect these vaccines to be available?
The CDC has urged local health departments to prepare for the rollout of COVID-19 vaccinations by November 1st, just days before the election. However, it's important to note that we have yet to see any interim results from ongoing trials.
This raises a crucial question: if vaccinations are to begin before the end of the year, which vaccine will lead the charge? Below, we will explore the seven vaccines in Phase 3 trials that may potentially be the frontrunners in this critical race.
Innovations in RNA Vaccines
Let's examine the innovative RNA vaccines developed by Pfizer and Moderna.
Traditional vaccines work by introducing viral components to teach the immune system to recognize and combat the virus. In contrast, RNA vaccines operate differently; they deliver RNA directly into your cells, prompting them to produce the viral protein independently. This method allows for rapid production, as RNA can be synthesized in large quantities without the need for complex bioreactors.
However, there are challenges associated with RNA vaccines. While initial data shows promising antibody responses, potential side effects remain unclear. Concerns have been raised about the possibility of autoimmune responses since the body's own cells produce the viral proteins. Additionally, these vaccines require extremely low storage temperatures, approximately -60 degrees Celsius.
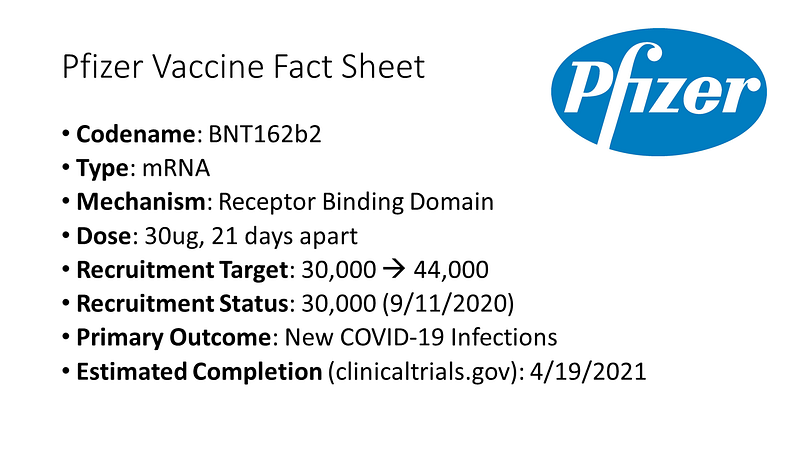
Pfizer's mRNA vaccine encodes the receptor binding domain of the SARS-CoV-2 virus, with modifications to enhance its immunogenicity. The trial initially aimed to recruit 30,000 participants but has expanded to 44,000 to ensure diversity. Efficacy data is expected in October, but safety data will take longer, which is crucial for FDA approval.

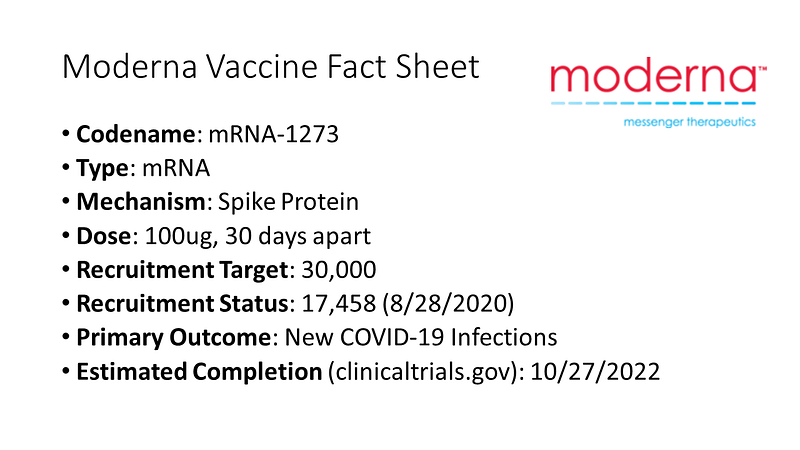
Similarly, Moderna's mRNA vaccine targets the spike protein and follows a comparable two-dose regimen. Their recruitment for a 30,000-person trial is reportedly over halfway complete.
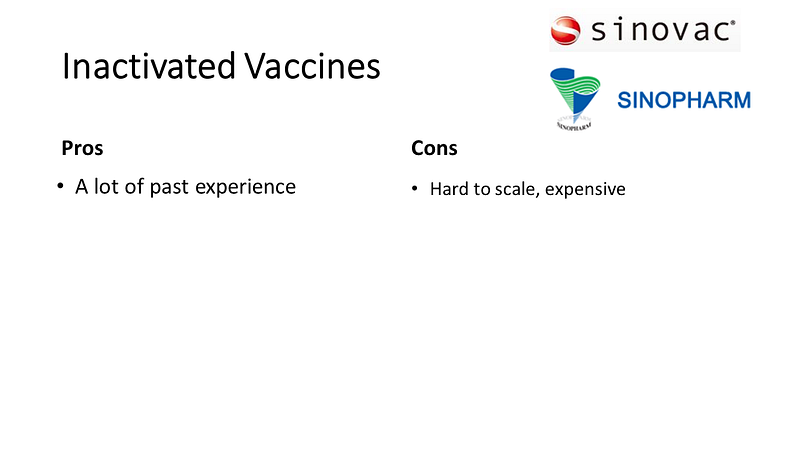
Established Methods: Inactivated Virus Vaccines
In contrast to RNA vaccines, inactivated virus vaccines from companies like Sinovac and Sinopharm use a more traditional approach, which is well-established but may struggle with large-scale production.
Sinovac's trial is unique in targeting a high-risk population of 8,870 healthcare workers, potentially allowing for a smaller yet adequately powered study. However, data on recruitment remains scarce.
Meanwhile, Sinopharm's vaccine has reportedly been approved for military use in China, having completed a large trial of 50,000 participants in the Middle East. Nevertheless, data transparency is lacking, and information about the vaccine itself remains vague.
Innovative Approaches with Adenovirus Vector Vaccines
Adenovirus vector vaccines, which utilize modified common cold viruses, represent another approach to vaccine development. These vaccines can elicit a strong immune response, but pre-existing antibodies against adenoviruses could hinder their effectiveness.
Three adenovirus vector vaccines are currently in Phase 3 trials. CanSinoBio uses an adenovirus type 5 vector to deliver the spike protein, but many individuals may already have antibodies against this vector.
AstraZeneca's vaccine employs a chimpanzee adenovirus, potentially mitigating the issue of pre-existing immunity. Their trial, which had reached 20,000 participants, faced a temporary halt due to a serious adverse event but has since resumed.

Lastly, the Gamaleya Research Institute in Russia is utilizing a combination of adenovirus types 5 and 26. This vaccine was controversially approved before completing Phase 3 trials, raising concerns about the integrity of the data.
Assessing the Road Ahead
As we evaluate these vaccines, no clear frontrunner has emerged. Preliminary data suggests similar antibody responses across the board, assuming the data can be trusted, especially from the Russian study.
The pressing question remains: will we see a vaccine available before the year's end? The answer hinges on several factors.
The FDA may grant emergency use authorization (EUA) for one or more of these vaccines. While this process can expedite vaccine availability, it is crucial that the Vaccines and Related Biological Products Advisory Committee, composed of independent scientists, reviews the data and gives their endorsement. An EUA lacking this approval would be concerning, but one supported by the committee could facilitate quicker vaccination efforts.
However, vaccine approval is just the initial step. The logistics of administering vaccines to the public is a more complex issue likely to extend into 2021.
Ultimately, the most significant milestone will be achieving widespread vaccination to restore normalcy. This requires not only logistical planning but also public trust. Transparent reporting of trial results, clear communication regarding potential side effects, and a strategy for monitoring unexpected events are essential.
In conclusion, overcoming this pandemic demands a unified approach grounded in scientific understanding. It is imperative that we learn from previous missteps to seize this critical opportunity.
This commentary was originally published on medscape.com.